-

No Cutting Necessary
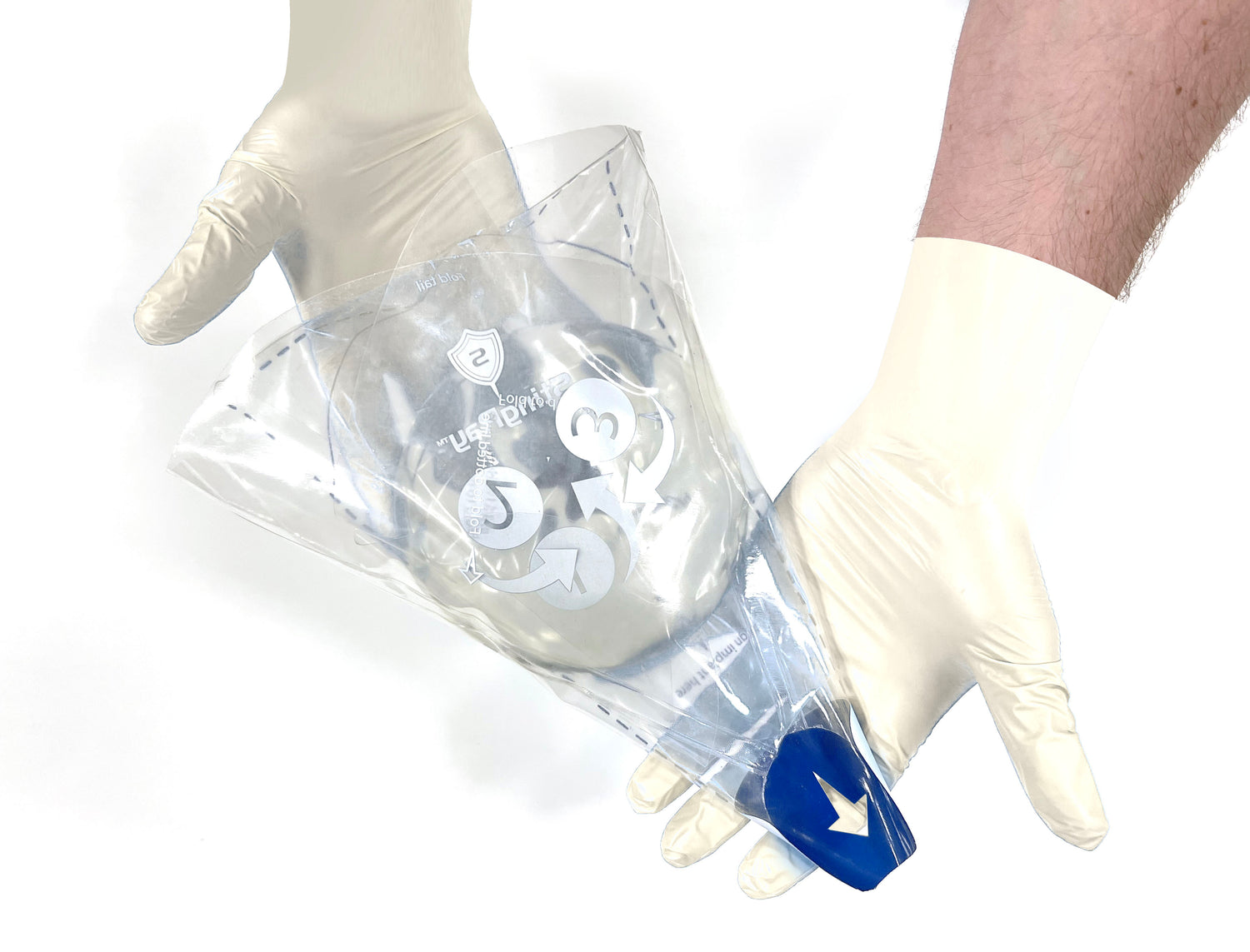
No scissors or cutting necessary.
-

Contact-Free Placement
Safely place implant without touching it.
-

One-Handed Technique
So simple it can be prepared with one hand.
StingrayTM is intended to facilitate the delivery of silicone gel implants by providing a shell-tissue interface with less friction during insertion of the implant. It is also intended to facilitate implementation of a contact free implant insertion.
Featured collection
-
StingRay (SR100) - 5 Pack
Regular price $1,000.00 USDRegular priceUnit price per -
StingRay (SR100) - 10 Pack
Regular price $2,000.00 USDRegular priceUnit price per -
 Sold out
Sold outStingRay (SR100) - 30 Pack
Regular price $6,000.00 USDRegular priceUnit price per
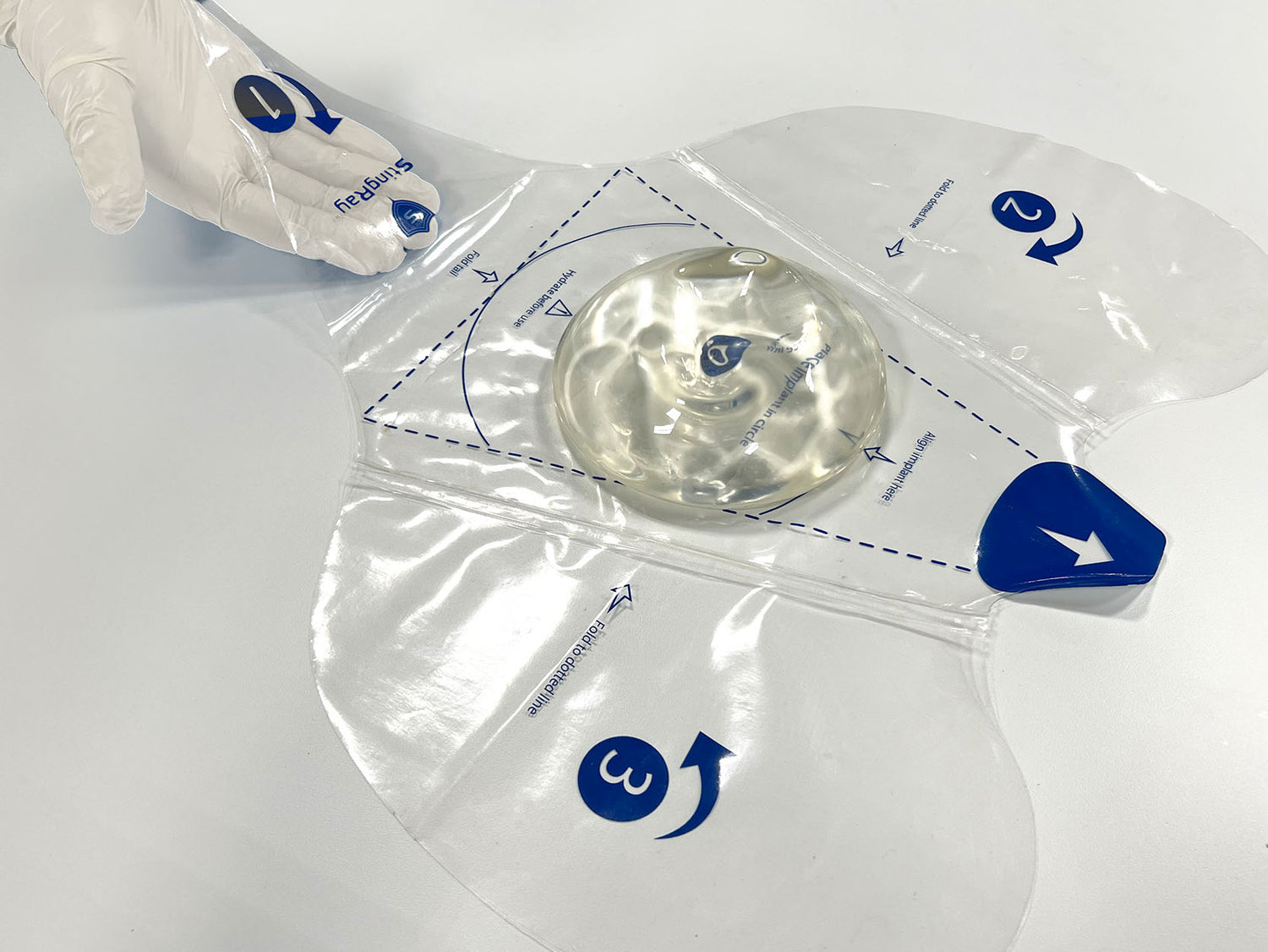
Easy Delivery Steps
-
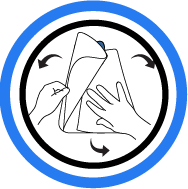
1. Unfold
Remove and discard the top paper liner.
-
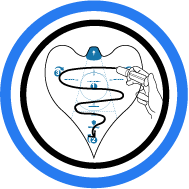
2. Hydrate
Using a sterile syringe or similar device, evenly distribute ...
-
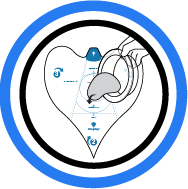
3. Place Implant
Gently place implant on the target spot.
-
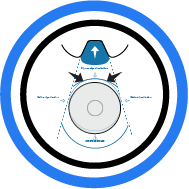
4. Align Implant
Align top of implant with guide lines as indicated ...
-
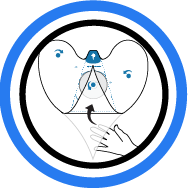
5. Wrap Tail
Reach under tail and flip up tail flap. Fold to the top edge ...
-
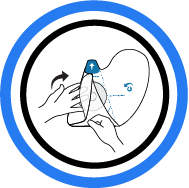
6. Wrap First Side
Fold over side flap. Fold to implant edge.
-

7. Wrap Second Side
Fold over other the side flap. Fold to edge of implant.
-
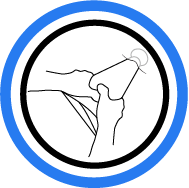
8. Deliver Implant
Insert tongue into incision. Manipulate and deliver ...
-
More Info
View StingRay’s full Instructions For Use document (IFU).

Frequently Asked Questions
How many times can the device be used?
The product is intended for single patient use only. Depending upon implant type and insertion location, the device can be configured to be used for two insertions on the same patient in a single procedure.
How can I learn how to use the device?
Please see the Instructions for Use Document to learn more about using StingRay.
How is the device sterilized?
The device is sterilized using Ethylene Oxide in sealed, double pouch packaging. Sterility is only maintained if the package seals are intact.
What if the package is damaged.
Do not use if package has been opened or damaged.
What should I do if the implant does not move easily through the device?
Do not use excessive force when delivering the implant. Stop if the implant does not advance using moderate pressure. Confirm that the incision and the surgical pocket are clear of obstructions and are appropriately sized for the implant. Check that the device is not twisted or creased in a manner that could obstruct the implant.
Who do I contact with questions about the device?
If you have any questions or would like additional information about StingRay, contact your StingRay sales representative or call StingRay's Research & Development Group (Vita Group LLC) at (612) 500-7433.
Where can I find the Instructions For Use (IFU)?
Please view and download the Stingray Instructions for Use.